The results can be summarized in a tabular form as follows;
Titration
| Burette readings | Pilot | 1 | 2 | 3 |
|---|---|---|---|---|
| Final reading (cm3) | ||||
| Initial reading (cm3) | ||||
| Volume used (cm3) |
MaVa = na MbVb nb
Volumetric analysis is the process of determination of concentration of a solution with the help of another solution of known concentration.
Volumetric analysis is experimental method of determination of volume of a solution of known concentration needed for a definite volume of another solution of unknown concentration.
Titration is the process of adding one solution to another solution until an indicator shows that the reaction is complete.
Titrant is the solution with a known concentration that will react with the analyte.
Analyte (titrand) is the solution with unknown concentration.
The end-point is the point in the titration at which the indicator changes colour.
Indicator is a chemical substance which changes colour at the end point.
The equivalence point (stoichiometric point) is the point at which chemically equivalent amounts/quantities of acid and base have reacted.
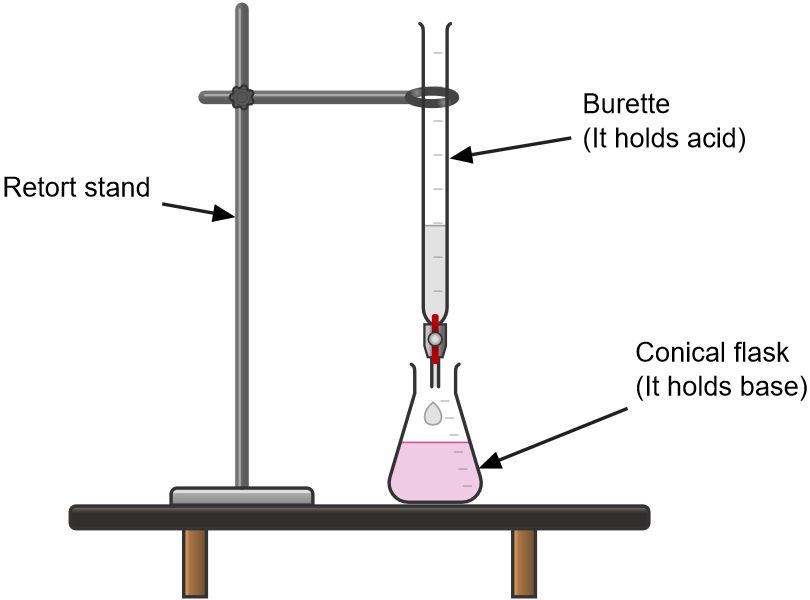
Burette
It is used to deliver variable volumes of a solution accurately. It is usually calibrated from 0 to 50cm3.
It should be washed with water and rinsed with the solution it is going to hold.
When reading a burette one should read exactly at the bottom of meniscus.
It is used to measure the fixed or specific volume of a solution accurately.
Like burette, it has to be washed with water and rinsed with the solution it is going to hold.
Pipette filler – used to fill a pipette. In case it is not present, the pipette may be filled by mouth suction, where care must be taken to not drink/swallow the solution.
Volumetric flask used to prepare a standard solution accurately. Like pipette it has a single calibration at its neck.
It is filled until the bottom of the meniscus rests on the line of the neck of the flask.
To determine the mass of the substance from the information given in the container
Mass = molarity x relative molecular mass x volume
% purity
McVc = MdVd where Mc = Molarity of concentrated solution Vc = Volume of the concentrated solution Md = Molarity of dilute solution Vd = Volume of the dilute solution.Vc = the volume to be taken from the bottle in order to get the desired molarity.
To calculate the molarity from the information given in the acid/base bottle
Molarity = % purity x density(ρ) x 1000cm3
molecular weight
Density sometimes is known specific gravity
Remember: ACID SHOULD BE ADDED TO WATER AND NOT WATER TO ACID
When concentrated acid is mixed with water, it produces a lot of heat. The heat produced is so much that if the water is poured directly to the acid, it can produce acid sprays which are harmful.

| Burette readings | Pilot | 1 | 2 | 3 |
|---|---|---|---|---|
| Final reading (cm3) | ||||
| Initial reading (cm3) | ||||
| Volume used (cm3) |
MaVa = na MbVb nb
| Acid-base | Example of acid & base | Indicator |
|---|---|---|
| 1. strong acid versus strong base | H2SO4 Vs. NaOH | Any indicator |
| 2.strong acid versus weak base | HCl Vs. NH4OH | Methyl orange |
| 3.weak acid versus strong | CHOOH Vs.KOH | Phenolphthalein |
| 4.weak acid versus weak base | C2H5COOH Vs. NH4OH | No suitable indicator |
| Indicator | Color of indicator in | ||
|---|---|---|---|
| Acid | alkali | Neutral | |
| Methyl orange | Pink | Yellow | Orange |
| Phenolphthalein | Colourless | Pink | Colourless |
| Litmus | Red | Blue | Blue or purple |
| Experiment | Pilot | 1 | 2 | 3 |
|---|---|---|---|---|
| Final reading (cm3) | 25.40 | 25.20 | 24.90 | 25.00 |
| Initial reading (cm3) | 0.00 | 0.00 | 0.00 | 0.00 |
| Volume used (cm3) |
(a) Average volume of acid used (cm3), Va:
Va = 25.20cm3 + 24.90cm3 + 25.00cm3
3
= 25.03cm3
Therefore, 25.03cm3 of the sulphuric acid neutralized 25cm3 0f 0.2M sodium hydroxide.
(b) H2SO4 + 2NaOH(aq) → Na2SO4 + H2O
na=1 nb = 2
(c) Molarity of acid (Ma) = ?
Volume of acid (Va) = 25.03cm3
Number of moles of acid (na) = 1
Molarity of base (Mb) = 0.2M
Volume of base (Vb) = 25cm3
Number of moles of base (nb) = 2
Frome acid-base mole ratio:
MaVa = na
MbVb nb
Ma = MbVbna
Vanb
Ma = 0.2mol/dm3 x 25cm3 1
25.03cm3 x 2
= 0.1mol/dm3
Therefore, the molarity of the acid is 0.1mol/dm3
(a) Balanced chemical equation:
2HCl(aq) + Na2CO3(aq) → 2NaCl(aq) + H2O(l) + CO2(g)
na = 2 nb = 1
Molarity of acid (Ma) = 2M
Volume of acid (Va) = 25cm3
Number of moles of acid (na) = 2
Molarity of base (Mb) = ?
Number of moles of base (nb) = 1
Volume of base (Vb) = 20cm3
From acid-base mole ratio:
MaVa = na
MbVb nb
Mb = MaVanb
Vbna
= 2mol/dm3 x 25cm3 x 1
20cm3 x 2
= 1.25mol/dm3
(b) The molar mass of Na2CO3 = (23x2) + 12 + (16x3)
= 106g/mol
Molarity = Concentration in g/dm3
Molar mass
Therefore:
Concentration in g/dm3 = Molarity x molar mass
= 1.25mol/dm3 x 106g/mol
= 132.5g/dm3
The relative atomic mass of an unknown element can be determined by titrating the acid or base containing the element with a standard solution.
(a)Data given:
Molarity of acid (Ma) = 1M
Number of moles of acid (na) = 1
Volume of acid (Va) = 10cm3
Molarity of base (Mb) = ?
Volume of base (Vb) = 25cm3
Number of moles of base (nb)= 2
From acid-base mole ratio:
From acid-base mole ratio:
MaVa = na
MbVb nb
Mb = MaVanb
Vbna
= 1mol/dm3 x 10cm3 x 2
25cm3 x 1
= 0.8mol/dm3
Therefore, the molarity of base is 0.8mol/dm3
(b) Molar mass = Concentration in g/dm3
Molarity
= 32g/dm3
0.8 mol/dm3
= 40g/mol
This means that
(X + 16 + 1) g/mol = 40g/mol
X = 40 - 17
= 23
(c) Since the relative atomic mass of X is 23, therefore the element X is sodium (Na).
Water of crystallization is the definite amount of water which some substances chemically combine with when they form crystals from their saturated solution.
Water of crystallization plays a vital role in the formation and properties of many inorganic compounds.
When certain compounds crystallize (form crystals from their saturated solution), water molecules become incorporated within their crystal lattice, forming stable bonds with the compound's ions or molecules. This water is referred to as water of crystallization.
The presence of water of crystallization affects the physical properties of compounds. For example, it can influence the color of crystals, as seen in compounds like copper sulphate, which appears blue due to the water molecules in its crystal structure.
The water of crystallization also affects the melting point and solubility of compounds, as the water molecules need to be released or incorporated in order to change the state or dissolve the compound.
The removal of water of crystallization can be achieved through processes such as heating or desiccation. By applying heat, the water molecules are evaporated, leaving behind an anhydrous compound. This process is often reversible, and upon exposure to moisture, the compound can regain its water of crystallization.
There are various experimental techniques which are used to determine the water of crystallization in a given compound, including titration method, where a solution containing water of crystallization is titrated with a standard solution such as a base or an acid.
Hydrate is the compound that contains water of crystallization. They are also known as hydrated compound. Example of acid that is hydrated is oxalic acid (COOH)2.2H2O and base is Na2CO3.10H2O.
The compound with no water of crystallization is usually called anhydrous compound.The amount of water in a hydrated acid or base can be determined through titration.
Concentration of hydrated compound(g/dm3) = Molar mass of hydrated compound Concentration of anhydrous compound (g/dm3) Molar mass of anhydrous compoundExample 01
The concentration in mol/dm3 is 0.625dm3
- Chemical equation:
Na2CO3 + 2HNO3 → NaNO3 + CO2 + H2O na = 2 nb = 1 given: Ma = 1 mol/dm3 na = 2 nb = 1 Va = 25cm3 Vb = 20 cm3 Mb = ? From MaVa = na MbVb nb Mb = MaVanb Vbna = 1mol/dm3 x 25cm3 x 1 20cm3 x 2 = 0.625mol/dm3
b. Concentration in g/dm3
Molarity = Concentration in g/dm3
Molar mass
Concentration in g/dm3 = Molarity x Molar mass
= 0.625mol/dm3 x 106g/mol
= 66.25g/dm3
The concentration in g/dm3 is 66.25g/dm3
The value of x can be calculated using the formula:
Concentration of hydrated compound(g/dm3) = Molar mass of hydrated compound
Concentration of anhydrous compound (g/dm3) Molar mass of anhydrous compound
179g/dm3 = (106 + 18x)g/mol
66g/dm3 106g/mol
Cancelling the units and dealing with numbers:
106 + 18x = 179 x 106
66
18x = 287.5 - 106
Dividing both sides by 18
18x = 181.5
18 18
x = 10
The value of x in the given compound is 10.
c. The formula for the compound is Na2CO3.10H2O