Sulphur dioxide
Sulphur dioxide is a colourless gas produced when sulphur or substances containing sulphur, for example, crude oil are burned in oxygen.
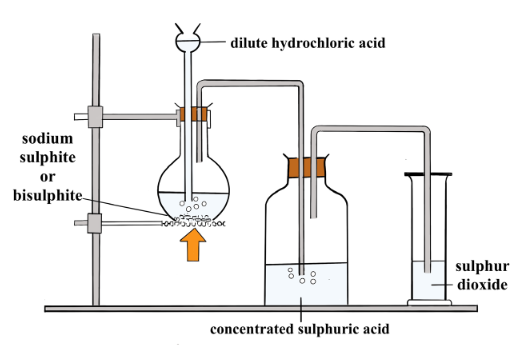
In the laboratory it is prepared by the action of dilute sulphuric acid on sodium sulphite.
Diagram Showing the Laboratory Preparation of Sulphur Dioxide
Na2SO3 + H2SO4 → Na2SO4 + H2O + SO2
The gas produced is passed through concentrated sulphuric acid to dry it and collected by downward delivery.
Properties of sulphur dioxide gas
(a) Physical properties
- It is a colourless gas with an irritating choking smell.
- It is a poisonous gas.
- It is readily soluble in water.
- It is denser than air.
- It is readily liquefied.
(b) Chemical properties
- Reaction with water.
It reacts with water to form sulphurous acid, H2SO3.
SO2 + H2O →H2SO3
Solution of sulphur dioxide slowly oxidizes to sulphuric acid in air.
SO2 + H2O + O2 → H2SO4
- It is a reducing agent. In the presence of water, sulphur dioxide behaves as a reducing agent
(a) 2FeCl3 + SO2 + 2H2O → 2FeCl2 + 2HCl + H2SO4
OR
Fe2(SO4)3(aq) + 5SO2(g) +2H2O(l) → 2FeSO4(aq) + 2H2SO4(aq)
Ionic equation:
2Fe3+(aq) + SO2(l) + 2H2O(l) → 2Fe2+(aq) + 4H+(aq) + SO42-(aq)
Iron is reduced from iron (III), Fe3+ (yellow) to iron (II), Fe2+(pale green).
(b) When sulphur dioxide is bubbled through acidified potassium dichromate(VI) solution, the solution changes from orange to green.
This is because; sulphur dioxide reduces chromium (VI) ions to chromium (III) ions.
K2Cr2O7(aq) + H2SO4(aq) + 3SO2(g) → Cr2(SO4)3(aq) + K2SO4(aq) + H2O(l)
Ionic equation:
3SO2(g) + Cr2O72- (aq) (orange) +2H+(aq) → 3SO42- (aq) + 2Cr3+ (green) (aq) + H2O(l)
(c) Chlorine is reduced to hydrochloric acid and sulphur dioxide oxidized to sulphuric acid
SO2 + Cl2 + 2H2O → H2SO4 + 2HCl
(d) In the presence of water, sulphur dioxide reduces concentrated nitric acid to nitrogen dioxide itself oxidizes to sulphuric acid.
SO2 + 2HNO3(aq) → H2SO4 + 2NO2
(e) Sulphur dioxide also reduces acidified potassium Manganate(VII) to manganese (II) sulphate.
The color changes from purple to colorless and the sulphur dioxide is itself oxidized to sulphuric acid.
2KMnO4(purple) + 5SO2 + 2H2O → K2SO4 + 2MnSO4(colourless) + 2H2SO4
Ionic equation:
5SO2 + 2MnO4- (aq)+ 2H2O → 5SO42- (aq) + 2Mn2+ (aq) + 4H+(aq)
- As oxidizing agent.
When sulphur dioxides reacts with a more reactive reducing agent than itself, it act as oxidizing agent.
Mg + SO2 → 2MgO + S
H2S + SO2 → S + 2H2O
- Reaction with oxygen
In the presence of catalyst reacts with oxygen to form sulphur trioxide.
SO2 (g)+ O2 (g)→ 2SO3(g)
- In solution form, it is a bleaching agent.
When sulphur dioxide dissolves in water it forms sulphurous acid. The sulphurous acid removes oxygen from the coloured substance and forms sulphuric acid. Some substances lose color when oxygen is removed from them.
SO2 + H2O → H2SO3
H2SO3 + coloured substance → [coloured substance – O] + H2SO4
(colourless substance)
The oxygen from the air may oxidize the reduced colourless substance slowly to its original coloured compound.
Differences between bleaching action of sulphur dioxide gas and chlorine gas
| Sulphur dioxide gas | Chlorine gas |
|---|
| Bleaches by reduction. | Bleaches by oxidation. |
The bleaching is temporary.
The bleached substance combines with oxygen from air over a period of time and regains its color. | The bleaching is permanent. |
Similarity:
Both sulphur dioxide and chlorine gases bleach only in the presence of moisture.
Tests for sulphur dioxide:
1. It turns acidified potassium dichromate solution from orange to green.
2. It turns purple acidified potassium permanganate solution colourless.
Uses of sulphur dioxide:
- Manufacture of sulphuric acid by contact process.
- As a refrigerant.
- As disinfectant.
- As bleaching agent.
Pollution effects of sulphur dioxide
| Produced | → power plants that use fossil fuels such as coal and diesel. |
|---|
→ industrial boilers |
→ exhaust emissions from motor vehicles |
| Effects | → impairment of respiratory function |
|---|
→ heart diseases |
→ cause acid rain: - destroys monuments and building especially those made of marble.
- when flows into streams, rivers, lakes changes the PH of the water and this affects the lives of aquatic organisms
|
