Non-metals and their Compounds
A brief introduction to non-metals
Chemical properties
- They react with oxygen to form acidic or neutral oxides
Acidic oxides : C + O2→ CO2
CO2 + H2O → H2CO3
Neutral oxides: CO, N2O and NO
- They do not react with dilute acids because non-metals are electron acceptor.
Therefore, they cannot supply electrons to H+ ions.
- React with other non-metals to form covalent compounds
P4 + 6Cl2 → 4PCl3
C + 2Cl2→ CCl4
- Displacement reactions.
More reactive non-metal displaces the less one from its aqueous solution.
NaBr (aq) + Cl2(g) → NaCl(aq) + Br2(l)
NaCl (aq) + F2(g) → NaF(aq) + Cl2(g)
KI (aq) + Cl2 (g) → KCl(aq) + I2(s)
Reactivity series for halogens: Fluorine > Chlorine > Bromie > Iodine > Astatine.
- They react with hydrogen to form hydrides.
H2 + S → H2S
N2 + 3H2 → 2NH3
- They are oxidizing agent.
On moving down the group in the periodic table the oxidizing property decreases.
Oxidizing propertiy for halogens: Fluorine > Chlorine > Bromie > Iodine > Astatine
(a)Chlorine
Occurrence:
Chlorine does not occur free in nature because it is very reactive.
In a combined state, it is mainly found in rock salt (NaCl)
Laboratory Preparation of Chlorine
Chlorine can be prepared in the laboratory by action of concentrated hydrochloric acid on oxidizing agents.
Oxidizing agents like potassium permanganate, manganese (IV) oxide, lead (IV) oxide and calcium hypochlorite.
Chlorine can be prepared by heating gently the mixture of manganese (IV) oxide and concentrated hydrochloric acid.
Laboaratory Preparation of Chlorine
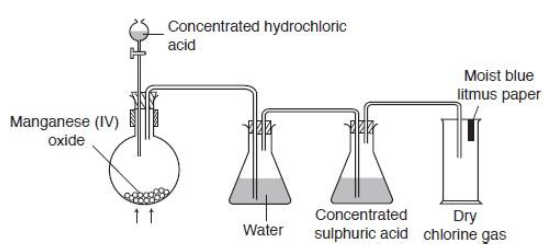
The chlorine gas evolved is passed through water to absorb hydrogen chloride gas that may be present. The chlorine gas is slightly soluble in water. The water gets saturated with chlorine ions after which the excess chlorine comes out.
Next the chlorine gas is bubbled through concentrated sulphuric acid to dry it.
Method of collection:
Chlorine is collected by upward displacement of air or downward delivery because it is denser than air.
Other reactions which produces chlorine:
- 2KMnO4 + 16HCl → 2KCl + 2MnCl2 + 8H2O + 5Cl2.
- CaOCl2 + 2HCl → CaCl2 + H2O + Cl2
- MnO2 + H2SO4 + 2NaCl → NaHSO4 + MnSO4 + H2O + Cl2
- K2Cr2O7+ 14HCl → 2KCl + 2CrCl3 + 7H2O + 3Cl2
Properties of Chlorine
(a) Physical properties
- It is greenish-yellow gas with a pungent irritating smell.
- It is denser than air.
- It is slightly soluble in water.
- It turns wet/damp/moist blue litmus paper red and then bleaches it.
(b) Chemical properties
- It reacts with water to form chlorine water.
Cl2 + H2O → HCl + HClO
- Displaces other less reactive halogens from their salts
2KBr + Cl2 → 2KCl + Br2
2KI + Cl2 → 2KCl + I2
- Chlorine is a good bleaching agent. It bleaches wet substances because it gives its oxygen atom (nascent oxygen) to a wet substance.
Some substances lose colour when oxygen is added to them.
Cl2 + H2O → HCl + HClO
Then HClO decomposes slowly
HClO → HCl + [O]
Coloured substance + [O] → Colourless substance.
This especially occurs when there is sunlight.
- As an oxidizing agent
Chlorine, oxidizes iron (II) chloride to iron (III) chloride
Cl2 + 2FeCl2(pale green) → 2FeCl3(reddish-brown)
Cl2 (g)+ H2S(g) → HCl(g) + S (yellow solid deposits)
Oxidizes sulphur dioxide solution to sulphuric acid
Cl2(g) + 2H2O(l) + SO2(g) → H2SO4(aq) + 2HCl(aq)
- Reacts with sodium hydroxides/potassium hydroxide
2NaOH + Cl2 → NaCl + NaClO + H2O
Dilute sodium
cold hypochlorite
6NaOH + 3Cl2 → NaClO3 + 3H2O + 5NaCl
Sodium
chlorate
The similar reaction occurs to potassium hydroxide.
- Reaction with ammonia gas
2NH3 + 3Cl2 → N2 + 6HCl
(limited)
NH3 + HCl → NH4Cl
Excess dense
white
fumes.
Overall equation of excess ammonia.
8NH3 + 3Cl2 → N2 + 6NH4Cl
Test of chlorine
It is a greenish-yellow gas which turns damp/moist/moist blue litmus paper red, then bleaches it.
Uses of chlorine
- It is used to sterilize water for domestic and industrial uses.
- It is used as a bleaching agent in textile industries.
- It is used to manufacture important chemicals such as chloroform, DDT, PVC.
- It used in the manufacture of hydrochloric acid.
- It is used in the manufacture of many organic solvents such as tetrachloromethane (CCl4) and trichloroethene. These can be used to remove grease and other non-polar substance
