It is used to separate the mixture of liquid and insoluble solid.
Examples of the mixtures which can be separated by this method are:
- sand and water
- rice and water
- chalk powder and water
- Allow the mixture to stand undisturbed for some time, allowing the solid to settle at the bottom.
- Carefully pour off the liquid portion without disturbing the settled solid.
- Collect the liquid and discard or further process the solid sediment.
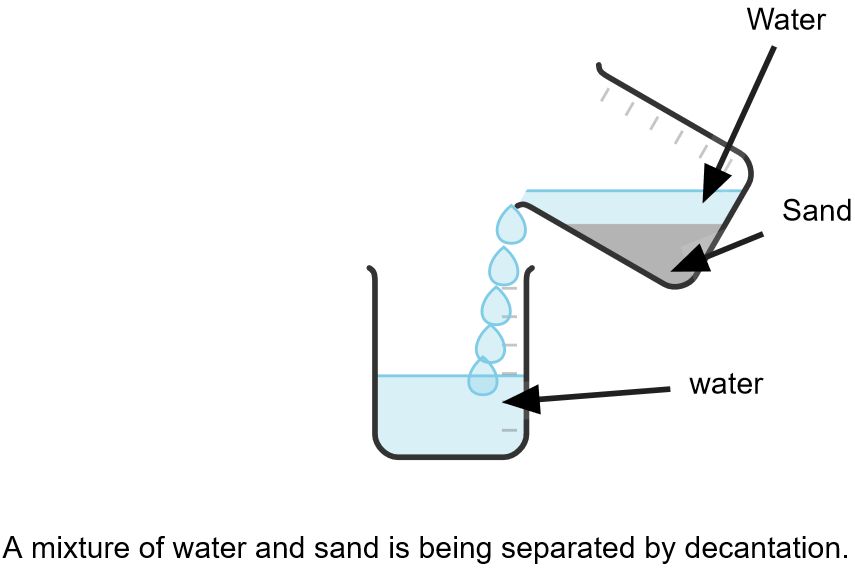
This process is effective if the solid particles are heavy and settle at the bottom of the liquid. But if solid particles are lighter (less dense) than liquid, they will remain suspended throughout the liquid. They can be forced to settle down by CENTRIFUGATION.
It is particularly useful for separating solid particles or precipitates from a liquid.
Here's the process:- Place the mixture in a centrifuge tube.
- Spin the tube at high speed in a centrifuge machine.
- The centrifugal force causes the denser components to move towards the bottom of the tube.
- Carefully remove the liquid without disturbing the small particles settled at the bottom.
- Collect the separated components by decantation or further processing.
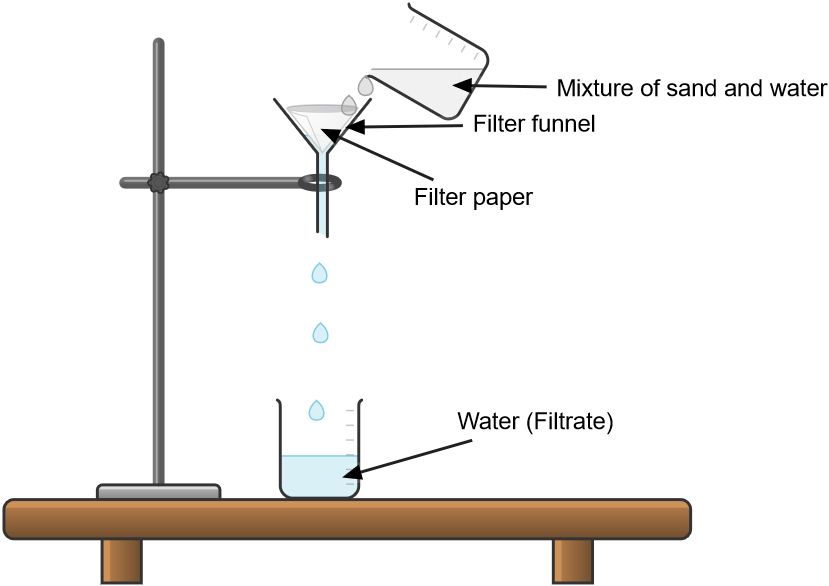
The liquid which passes through the filter paper is called filtrate
The solid that remains on the filter paper is called residue
Illustration
a. Set up a funnel with filter paper or a porous material in it.
b. Place a container beneath the funnel to collect the liquid (filtrate).
c. Pour the mixture into the funnel.
d. The liquid will pass through the filter paper, while the solid particles will be retained on the filter.
e. Carefully remove the solid from the filter paper to obtain the separated components.
For example, if you have a mixture of sand and water, filtration can be used to separate the sand particles from the water.
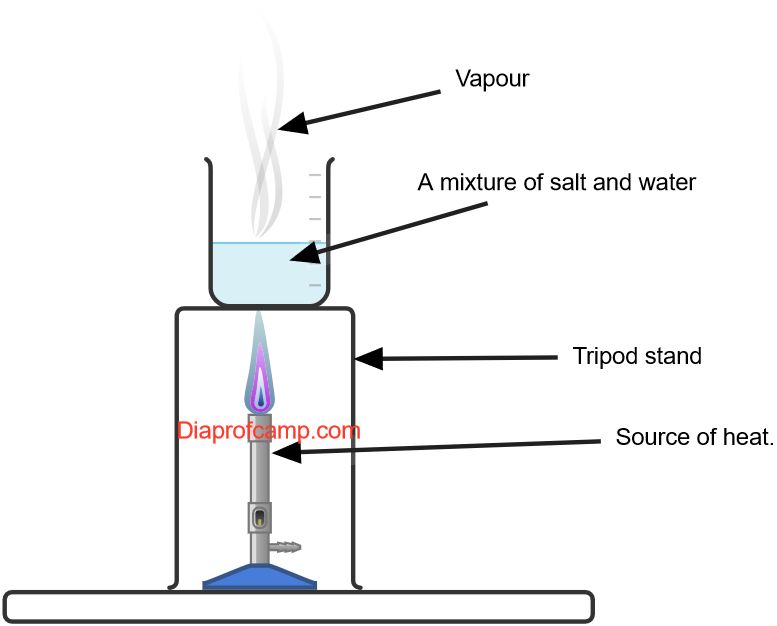
It takes advantage of differences in boiling points between the solvent and the solute.
Here's how it works:- Pour the mixture into a container.
- Heat the container, allowing the liquid component (solvent) to evaporate.
- As the liquid evaporates, the solid component (solute) will be left behind.
- Collect the solid once all the liquid has evaporated.
It is particularly useful when the components have significantly different boiling points.
Examples of mixtures which can be separated by this method are
- Salt solution.
- Copper(II) sulphate solution
- Muddy water
To carry out the process:
a. Heat the mixture in a distillation flask.b. The component with the solvent will vaporize.
c. The vapor is then condensed back into a liquid by cooling it in a condenser
d. Collect the condensed liquid, which represents the separated component.
It is used to separate the miscible liquids which have different boiling points.
Some mixtures which can be separated by fractional distillation are:
- Crude oil (petroleum)
- Air
- Ethanol(alcohol) and water
To perform fractional distillation, we require specific equipment:
- Distillation flask: Holds the mixture to be separated.
- Fractionating column: A long column packed with materials such as glass beads or metal pieces, providing a larger surface area for vaporization and condensation.
- Condenser: Converts the vapor back into liquid form using cold water.
- Receiver flask: Collects the separated components
Setup and Operation
The setup involves connecting the distillation flask to the fractionating column, condenser, and receiver flask.
The distillation flask is heated gradually, and the temperature is carefully controlled to achieve the desired separation. Fractional Distillation Process
- Heating and Vaporization The mixture is heated, gradually raising the temperature. The component with the lowest boiling point vaporizes first, rising up the fractionating column.
- Fractionation in the Column As the vapors rise through the fractionating column, they come into contact with the packing material. The packing provides a large surface area for condensation, allowing the higher boiling point components to condense and flow back down into the flask.
- Condensation and Collection The vapors that reach the top of the column enter the condenser, where they are cooled and converted back to liquid form. The condensed liquid is collected in the receiver flask, separate from the original mixture.
- Multiple Distillation Cycles
Immiscible liquids are liquids that do not dissolve in each other and form separate layers when mixed.
The principle of funnel separation relies on the difference in densities of the immiscible liquids.
The liquid with the higher density will form the bottom layer, while the liquid with the lower density will form the top layer.
Common examples of immiscible liquids include:
- Cooking oil and water
- Hexane and water
- Kerosene and water
Process Overview:
- Addition of Mixture: The mixture of immiscible liquids is poured into the separating funnel.
- Settling: The mixture is allowed to settle, forming two distinct layers.
- Draining: The stopcock is opened to drain the bottom layer into a separate container.
- Separation: The top layer is then collected after the bottom layer has been removed.
It relies on differences in solubility, adsorption, or other interactions between the components and the phases. Here's a basic outline of the process:
- Apply the mixture to a stationary phase, which is often a solid or a liquid absorbed on a solid support.
- Introduce a mobile phase, which can be a liquid or a gas, which carries the components through the stationary phase.
- The different components of the mixture will interact differently with the stationary and mobile phases, leading to separation.
- The separated components can be collected and analyzed.
Magnetic separation is a method that exploits the magnetic properties of certain substances to separate mixtures. This technique is often used to separate magnetic materials such as iron from a mixture
Common substances which can be attracted by magnet are nickel,iron, cobalt steel (NICS)etc.
Some mixtures which can be separated by magnetic separation method are:
- Iron and sand
- iron and sulphur
- Place the mixture in a container or on a surface.
- Bring a magnet close to the mixture or pass it through the mixture.
- The magnetic components will be attracted to the magnet, allowing for their separation from the non-magnetic components.
- Carefully remove the magnetic components from the magnet. Magnetic separation is commonly used to separate a mixture containing iron filings or other magnetic substances.
It can be used to separate a mixture where one component has sublimation properties.
Some of the substances which has sublimation properties are:
- Iodine
- Ammonium chloride
- Solid carbon dioxide (dry ice)
- iron(III) chloride
Some of mixtures which can be separated by this method are:
- Iodine and sand
- Ammonium chloride and sodium chloride (common salt)
- Ammonium chloride and chalk powder
- Heat the mixture gently.
- The component with sublimation properties will directly convert from a solid to a gas, leaving behind the other component.
- Collect the gas by cooling it and condensing it back into a solid form.
- The separated components are now obtained. An example of sublimation is the separation of iodine from a mixture of iodine and sand.
Solvent extraction involves separating components based on their solubility in different solvents.
By adding a suitable solvent, the desired component can be selectively dissolved, while the other components remain insoluble.
After dissolved in such a solvent, the mixture of the desired component and solvent can be separated by evaporation.
Illustration- Add the mixture to a container.
- Introduce a suitable solvent that selectively dissolves the desired component.
- Stir or shake the mixture to facilitate the transfer of the component into the solvent.
- Allow time for the separation to occur.
- Carefully separate the solvent (containing the desired component) from the mixture.
- The separated component can be obtained by evaporating the solvent.
It involves passing the mixture through the holes or openings of a sieve or mesh which allows smaller particles to pass through while retaining larger ones.
It is a traditional agricultural method used to separate grain from chaff or unwanted substances. The important principle is that these unwanted substances should be lighter than grains in order to be carried away with wind and leave the grains behind.
Application of methods of separating mixtures
- Obtaining the components of air such as oxygen.
- Alcohol production
- Forensic analysis- identifying the components of drugs.
- Food industry
- Recycling magnetic materials such as iron and steel.
- Mining industry uses this method to separate magnetic minerals such as magnetite from others.
Filtration
It is used in water treatment plants to remove suspended particles or impurities.Evaporation
It is widely used in the production of salt from seawater.Fractional distillation
- Petroleum refining.