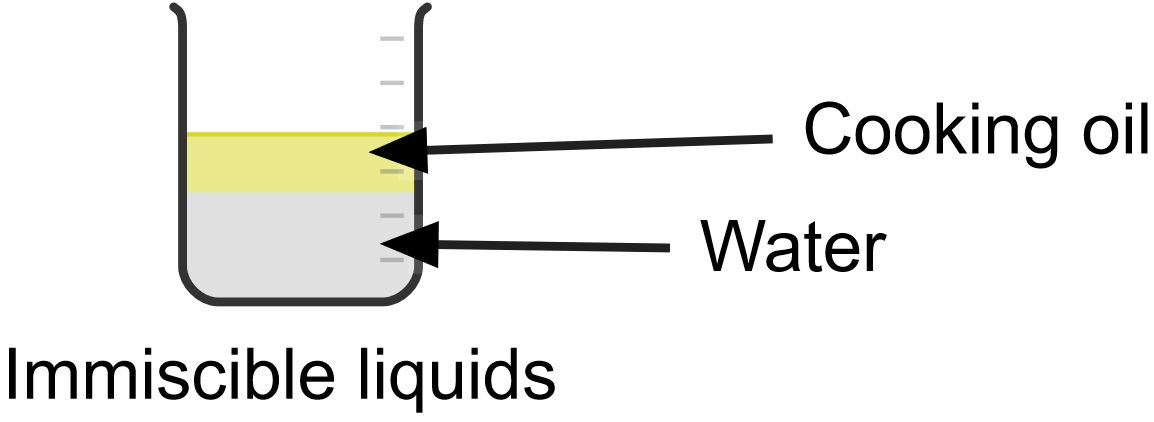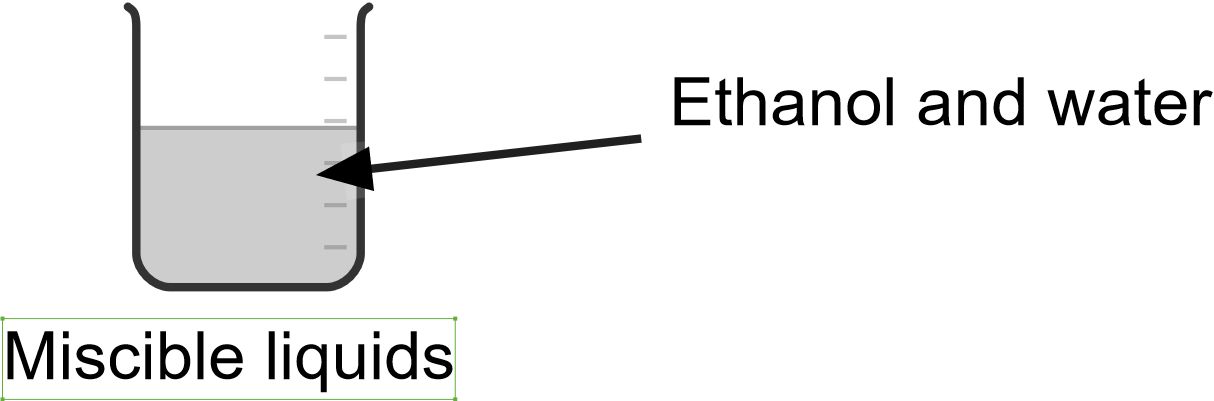
Compound is a pure substance made up of two or more elements in a chemical combination. The combinations are always in a fixed ratio. Examples of compounds are carbon dioxide and water.
Mixture is a physical combination of two or more substances in any ratio. It can also be defined as a substance which consists of two or more elements or compounds not chemically combined together. Examples of mixtures are sand and water, oil and water.
| Compounds | Mixtures |
|---|---|
| The constituent elements cannot be separated by physical methods. | The components can be separated from one another by physical methods. |
| Chemical changes are involved when compounds are formed. | No chemical change occurs when mixtures are formed. |
| The components cannot be seen separately. | Components may be seen separately. |
| They have always fixed composition by mass of the elements. | Mixtures may vary in composition. |
| The properties of compounds are very different from those of the individual elements. | The properties of mixtures are those of the individual components. |
Some solid substances are melted and mixed together then allowed to cool.
If this involves metals to metals or metals and non-metals, the resulting mixture is called alloy.
Alloy is a homogeneous mixture of two or more metals or of metals and non-metals.
Examples of alloys| Alloy | Composition |
|---|---|
| Brass | Copper + zinc |
| Bronze | Copper + tin |
| Stainless steel | Iron + chromium + nickel |
Solute is the substance that dissolves in solvent while solvent is the substance that dissolves solute.
Solvent is the component of the solution that is present in large amount in a solution.
SOLUTE + SOLVENT → SOLUTION
Examples of solutions
Unsaturated solution is a solution that can dissolve more solute at a given temperature.
Saturated solution is a solution that can dissolve no more solute at a given temperature.
Super saturated solution is a solution that contains more solutes than it can hold at a given temperature.
Gaseous solution example air, water gas and producer gas
Solid solution example all alloys such as brass, bronze and stainless steel
Liquid solution example ethanol in water, petrol and kerosene
There are two types of solventsSubstance can dissolve into these solvents according to the simple rule ‘like dissolves like’.
- polar solvent
- non-polar solvent.
A suspension is a liquid containing small particles of solids which are spread throughout it and the solid particles settle on standing.
orSuspension is the heterogeneous mixture where solid particles are dispersed throughout a liquid and can settle at the bottom of container if left undisturbed.
Examples are| Solutions | Suspensions |
|---|---|
| They are homogeneous mixtures. | They are heterogeneous mixtures. |
| They are transparent or clear. | They are opaque or not clear. |
| Solute particles completely dissolved in a solvent. | Solute particles settle if the suspension is undisturbed. |
| Components can be separated by evaporation. | Components can be separated by filtration. |
Colloid is the mixture where very small particles of one substance are evenly spread throughout another substance.
Colloids appear very similar to solutions but the components in colloids are not dissolved in each other.
Colloid is also similar to suspension but the difference being that the particles in colloids do not settle at the bottom even when left undisturbed.