Matter
Matter is anything that has mass and occupies space.
Matter is everything that is found within our environment.
All matter is made up of tiny (small) particles invisible to our naked eyes. These particles are atoms, molecules or ions.
States of Matter
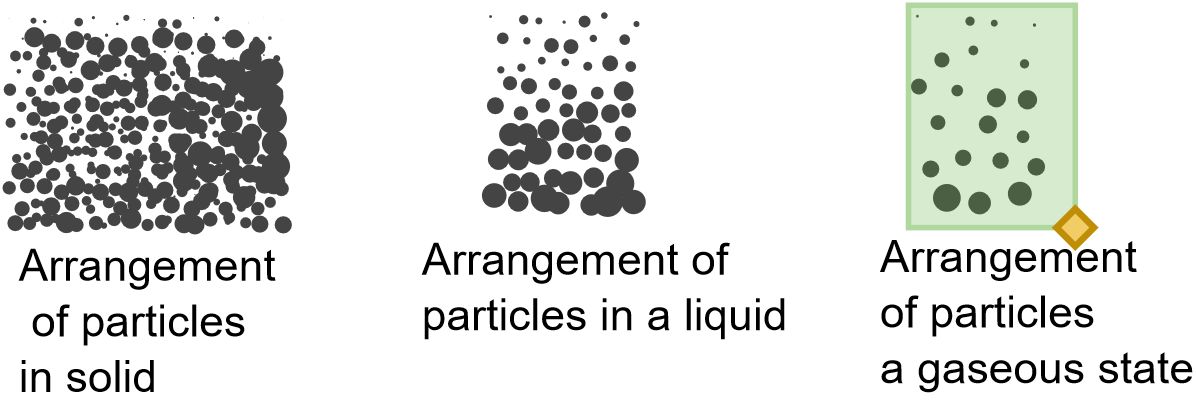
There are three states of matter.
→The state of matter depends largely on temperature and pressure.
→The liquids and gases are usually classified as FLUIDS.
Solid state
The solid matter have the following important characteristics:
- They have definite shape and definite volume .
- They are hard and incompressible (they cannot be squeezed easily into a smaller volume)
They possess these characteristics because the particles that make up these solids are packed closely together and arranged in the specific order
Examples of solids are ice, stone, chair and bottles.
Liquid state
Liquid matter have the following key characteristics
- They have definite/specific/fixed volume.
- They flow. Because particles are held in no specific arrangement and are free to move past one another.
- They take the shape of the container holding them.
- They are incompressible. Because their particles are packed closely together.
Examples of liquids are water, petrol, kerosene and ethanol.
Gaseous state
- Particles have no definite volume.
- They flow.
- They are compressible, because of the spaces between their particles.
- Take the shape of their container.
They have these properties because their particles are free to move apart to fill the volume of the container.
Examples of gases are oxygen, carbon dioxide and nitrogen.
| Solid | Liquid | Gaseous |
|---|
| Have a definite shape | Have no definite shape | Have no definite shape |
| Have a fixed volume | Have a fixed volume | Have no fixed volume |
| Do not flow easily | Flow easily | Flow easily |
| Particles are closely and tightly packed | Particles are closely but not tightly packed | Particles are not closely packed. |
| No free space between particles | Little free space between particles | Lots of free spaces between particles. |
| They are incompressible | They are incompressible | Can be compressed |
| Particles vibrate in their fixed position | Particles can slide past one other | Particles are in constant random motion since they are far away from each other |
Changes in States of Matter
The physical states of matter can be changed by - Changing temperature.
- Changing pressure.
In case of temperature, changes of matter occur when matter absorbs or loses energy.
Pressure is applicable for gaseous state of matter. For example when pressure is applied to a gas it can cause the gas particles to come closer together and condense into a liquid.
The main processes involved in changing matter are:
MELTING
This is the process of changing a solid to a liquid. The constant
temperature at which a solid changes into a liquid is called melting point
SOLID → LIQUID
FREEZING
The process by which a liquid changes to a solid is freezing.
The constant temperature at which a liquid changes into a solid is referred to as
freezing point.
LIQUID → SOLID
VAPOURIZATION
Vapourization refers to the process by which liquid substance changes into a gas. It can occur in two forms:
The constant temperature at which a liquid changes into a gas is the boiling point.
| Evaporation | Boiling |
|---|
| Occurs at all temperatures below boiling point. | Occurs at specific temperature (boiling point) |
| Occurs on the surface of the liquid. | Occurs throughout the entire liquid. |
| It is a slow process. | It is a fast process. |
| Bubbles are not necessarily formed. | Bubles are formed. |
| It require very minimum amount of heat. | It needs high amount of heat in order to take place. |
LIQUID → GAS
CONDENSATION
The process by which a gas changes into a liquid is called condensation.
GAS →LIQUID
SUBLIMATION
Sublimation is the process by which a solid changes directly to a gas.
SOLID → GAS
DEPOSITION
Deposition is the process by which a gas changes directly to a solid.
GAS → SOLID
Kinetic Theory of Matter
Kinetic theory of matter states that "matter is made up of tiny (small) particles (atoms, molecules, ions) that are in constant motion".
The main points of the theory are:
- Matter is made up of tiny particles called molecules, atoms or ions.
- These particles are always moving and can collide with each other. In which:
- Particles in a solid vibrate in their fixed position.
- Particles in liquid move/slide past each other.
- Particles in any gas are far apart from each other, therefore they are free to move in any direction with relatively high speeds.
- The particles are held together by attractive forces between them. These forces are known as intermolecular forces.
- In solid, there is strong attractive forces between its particles.
- In liquid, there is moderate forces of attraction.
- Gases have weak forces of attraction between them, this allows them to expand and occupy the entire volume of their container.
- As the temperature increases, the particles move faster and have more kinetic energy.
The Proof of Kinetic Theory of Matter
1. Brownian Motion:
Brownian movement/motion is the random movement of particles suspended in a fluid. A fluid means liquid or gas. This movement is caused by collision between the solid particles and the moving particles in the fluid.
Observation: In 1827, Robert Brown observed that tiny pollen grains suspended in water moved in a random, zigzag fashion. This motion was later named Brownian motion.
Explanation: The kinetic theory explains this by proposing that the pollen grains are constantly bombarded by the randomly moving water molecules. The collisions with these invisible water molecules cause the visible pollen grains to move erratically. This provided strong visual evidence for the existence of constantly moving particles.
Another brownian motion can be observed in the movement of smoke particles in air.
2. Diffusion:
Diffusion is the movement of particles from a region of higher concentration to a region of lower concentration.
Observation:
- When you spray perfume in a room, the scent eventually spreads throughout, even without air currents.
- Similarly, if you drop a dye into water, it slowly spreads out until the entire solution is uniformly colored.
Explanation: The kinetic theory explains this by stating that the perfume or dye molecules are in constant motion. They collide with other air or water molecules, spreading out from an area of high concentration to one of lower concentration. This random motion of particles leads to the observed diffusion.
Factors affecting the rate of diffusion
- Temperature
Higher temperature: Increases the kinetic energy of particles, leading to faster diffusion.
Lower temperature: Decreases kinetic energy, resulting in slower diffusion.
- Density of Particles
Lower density: Gases with lower density diffuse faster than those with higher density.
- Concentration Gradient
Steeper gradient: A larger difference in concentration between two areas leads to a faster diffusion rate.
- Surface Area
Smaller particles: Smaller particles diffuse faster than larger particles of the same mass due to their increased surface area-to-volume ratio.
Changes of states of matter and Kinetic theory
Kinetic theory "All substances are composed of particles (atoms, ions or molecules) that are in constant motion".
Melting. When a solid is heated, the particles gain kinetic energy and start to vibrate more vigorously. As the temperature increases, the intermolecular forces holding the particles in fixed positions weaken, allowing the particles to move more freely and change into a liquid state.
Vapourization.. When a liquid is heated further, the particles gain even more kinetic energy, overcoming the intermolecular forces holding them together. The particles separate and move randomly in all directions, forming a gas.
Condensation. When a gas is cooled, the particles lose kinetic energy and move more slowly. The intermolecular forces become stronger, causing the particles to come closer together and form a liquid.
Freezing. As a liquid is cooled further, the particles lose more kinetic energy, and the intermolecular forces become strong enough to hold the particles in fixed positions, forming a solid substance.
PHYSICAL AND CHEMICAL CHANGES
Matter can undergo two types of changes:
- Physical change
- Chemical change
Physical change
Physical change is the change whereby no new substance is formed. It does not affect the composition of the matter.
→Some examples of processes that involve physical changes are:
- Crushing of chalk into powder
- Melting of ice
- Burning of candle wax
- Boiling of water
- Dissolving salt or sugar in water.
- Cutting a substance.
- Magnetization/demagnetization of iron.
Chemical change
Chemical change is the change whereby new substance is formed. It can affect the composition of matter.
- Ripening of fruits .
- Burning of paper.
- Souring of milk.
- Rusting of iron.
- Burning of fuel.
- Photosynthesis
- Digestion of food
NOTE
The chemical change is also known as chemical reaction.
Differences between physical and chemical Changes
| Physical Changes | Chemical Changes |
|---|
| Affect only physical properties of matter. | Affect both physical and chemical properties of matter. |
| Are temporary changes. | Are permanent changes. |
| Are easily reversible. | Are irreversible. |
| No new substance is formed | New substances are formed. |
| No energy is produced or absorbed. | Energy is produced or absorbed. |
Elements
Matter is made up of substances called elements.
Element is a pure chemical substance which cannot be split into simpler substances by a simple chemical process.
There are so many elements now discovered.
Names and Symbols of Elements
Chemical symbols are short representations of the names of elements.
Criteria for Assigning Chemical Symbols
- An element may be represented by a chemical symbol that is derived from the first letter of its English name.
Examples:
| Name | Chemical symbol |
|---|
| Boron | B |
| Carbon | C |
| Fluorine | F |
| Hydrogen | H |
- An element may be represented by the initial letter and another letter either the second or third letter from the English name.
In this case, the first letter is always a capital while the second letter is small letter.
Examples:
| Name | Chemical symbol |
|---|
| Aluminiun | Al |
| Beryllium | Be |
| Calcium | Ca |
| Magnesium | Mg |
| Manganese | Mn |
| Silicon | Si |
| Cobalt | Co |
- Some elements derive their chemical symbols from Latin names.
| English name | Latin name | Chemical symbol |
|---|
| Copper | Cuprum | Cu |
| Gold | Aurum | Au |
| Iron | Ferrum | Fe |
| Sodium | Natrium | Na |
| Potassium | Kalium | K |
| Lead | Plumbum | Pb |
| Mercury | Hydrargyrum | Hg |
| Silver | Argentum | Ag |
| Tin | Stannum | Sn |
| Antimony | Stibium | Sb |
| Tungsten | Wolfram | W |
Metals and Non-metals
Elements can be classified into metals and non-metals.
- Examples of metals are aluminium, sodium, copper, zinc, mercury and magnesium.
- Mercury is the only metal which is in liquid state. The other metals are in solid state.
- Examples of non-metals are sulphur, carbon, bromine, chlorine and phosphorus.
- Bromine is the only non-metal which is in liquid state; the other non-metals are in solid and gaseous state.
Physical differences between metals and non-metals
| Metals | Non-metals |
|---|
| They are good conductors of electricity. | They are poor conductors of electricity. |
| They are good conductors of heat. | They are poor conductors of heat. |
| They have melting points and boiling points. | They have low melting points and boiling points. |
| They are ductile. This means that they can be drawn into thin wires. | They are not ductile. They cannot be drawn into thin wires. |
| They are malleable. This means that they can hammered into thin sheets. | They are not malleable. |
| They are sonorous. That is they produce sound when hit with something | They are not sonorous. |
| They are lustrous. They have shining surfaces when cut. | They are not lustrous. |
| They have high tensile strength. That is the ability to withstand stress. | They have low tensile strength. |
Volume of a substance is the amount of space the substance takes up.

