Hydrogen chloride gas
Hydrogen chloride gas is a compound of chlorine and hydrogen. Its formula is HCl.
Laboratory preparation of Hydrogen chloride gas
Hydrogen chloride gas can be prepared in the laboratory by reacting rock salt (sodium chloride, NaCl) with concentrated sulphuric acid.
Diagram Showing Laboratory Preparation of Hydrogen Chloride
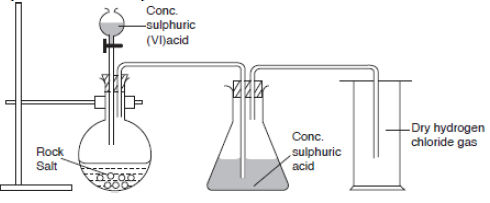
NaCl(s) + H2SO4(l) → NaHSO4(s) + HCl(g)
The gas is passed through bottle containing concentrated sulphuric acid to dry it.
It is collected by downward delivery or upward displacement of air.
PROPERTIES OF HYDROGEN CHLORIDE GAS
(a) Physical properties of hydrogen chloride gas
- Hydrogen chloride gas is a colourless gas.
- It has a pungent choking smell and the sharp taste of acids.
- It is an acidic gas that is turns moist blue litmus paper red.
- It is denser than air.
- It is highly soluble in water. Its solubility is usually demonstrated by Fountain Experiment.
(b) Chemical properties of hydrogen chloride gas
- It reacts with ammonia gas to give the dense white fumes of ammonium chloride.
NH3(g) + HCl(g) → NH4Cl (dense white fumes)
- It dissolves in water to form hydrochloric acid.
HCl(g) + H2O → HCl(aq)
- Reacts with silver nitrate solution to give white precipitate
HCl(aq) + AgNO3 → AgCl(s) + HNO3(aq)
- Reacts with oxidizing agents to liberate chlorine gas
PbO2 (s)+ HCl (g)→ PbCl2(s) + 2H2O(l) + Cl2(g)
Tests for hydrogen chloride gas
- It forms dense white fumes with ammonia.
- It forms white precipitate with acidified silver nitrate solution
- It forms misty fumes in moist air.
Uses of hydrogen chloride gas
- It is used in analytical chemistry.
- Hydrochloric is used to remove rust (oxide) from iron.
Fe2O3 (s)+ 6HCl (aq) → 3FeCl2 (aq) + 3H2O(l)
- It is used in the preparation of chloride salts.
- It is used in the production of organic compounds such as PVC.
