HYDROGEN
Hydrogen is the lightest and the most abundant element in the universe.
Hydrogen gas is lighter than air and therefore rises in the atmosphere.
Hydrogen is a very reactive element. This is why it is found in combination with many other elements.
Laboratory Preparation of Hydrogen
- Reaction of dilute acids with some metals.
- The reaction of water with certain metals.
- The reaction of water with hot carbon.
- The electrolysis of water.
The commonest method of preparation is by the action of dilute acids on metals.
Acids commonly used: sulphuric acid, hydrochloric acid.
Metals frequently used: magnesium, zinc, iron and calcium.
Diagram Showing the Laboratory Preparation of Hydrogen
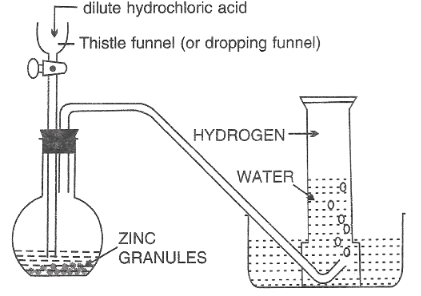
Word equation:
Zinc + hydrochloric acid →zinc chloride + hydrogen
Molecular equation:
Zn + 2HCl →ZnCl2 + H2
Hydrogen can be collected by downward displacement of water because it is slightly soluble in water.
Hydrogen can also be collected by upward delivery or downward displacement of air because it is less dense (lighter) than air.
Properties of Hydrogen
- Physical properties
- It is tasteless, colourless and odourless.
- It is lighter than air.
- It is slightly soluble in water.
- It does not support combustion.
- Chemical properties.
- Reacts slowly with oxygen to produce water.
- It is a good reducing agent.
- It combines with some metals to form metal hydride.
- It is neither acid nor basic.
- It does not react with other elements at room temperature.
Test of hydrogen/Identity test of hydrogen gas
When a burning splint is introduced into the gas jar containing hydrogen gas, the hydrogen gas burns with a “pop” sound.
Or restate:
Hydrogen gas produces a "pop" sound when a burning splint is brought near it.
Illustrate
- Place a small amount of the hydrogen sample in a test tube.
(This can be achieved by putting zinc granules in test tube and adding dilute acid eg. hydrochloric or sulphuric acid (2mol/dm3). Then cover the mouth of the test tube to prevent the escape of the gas.
- Bring a lighted wooden splint or match near the mouth of the test tube.
- If hydrogen is present, it will make a "pop" sound as the hydrogen gas ignites. (The sound is produced by the combustion of hydrogen with oxygen).
Industrial production of hydrogen
- Electrolysis of acidulated water.
- The action of hydrocarbons on steam.
CH4 + H2O →CO + H2. This is known as steam methane reforming (SMR)
Uses of Hydrogen
- It is used in the manufacture of ammonia.
- It is used in the manufacture of margarine. Hydrogen is bubbled through liquid oil with nickel as a catalyst, thereby hardening the oil. The process is called hydrogenation.
- It is used to produce the oxy-hydrogen flame.
- It is used to prepare water gas which is used as a fuel.
- It is used in the manufacture of hydrochloric acid.
- It is used to fill weather balloons.
- It is used as a fuel.
Relationship between uses hydrogen and its properties
| Uses | Properties |
| Manufacture of ammonia | Readily combines with elements eg. nitrogen |
| Production of oxy-hydrogen flame | It is highly flammable. |
| Manufacture of hydrochloric acid | Readily reacts with other chemical substances. |
| Preparation of water gas | It is highly flammable |
| In weather balloons | It is lighter than air. |
| Manufacture of margarine | It is a reducing agent. |
Oxidation and reduction
| Oxidation | Reduction |
|---|
| Addition of oxygen to a substance. | Removal of oxygen from a substance. |
| Removal of hydrogen from a substance. | Addition of hydrogen to a substance. |
| Removal of electron from a substance. | Addition of electron to a substance. |
| Increase in oxidation state (oxidation number) of the substance. | Decrease in oxidation state (oxidation number) of the substance. |
Reducing agent is a substance that causes reduction to another substance.
Oxidizing agent is a substance that causes oxidation to another substance.
