Heat Sources and Flames
There are some experiments which need heat in order to get good results. Heat is also required to make some reactions (chemical changes) to take place fast.
The choice of heat sources can depend on:
There are different sources of heat in chemistry laboratory. However not all are good for use. Factors govern the choice of source of heat include;
- Availability of itself or its fuel
- Cost
- Storage Space
- Handling
- Amount of heat it produces.
Why heat source is important in the laboratory?
- To sterilize laboratory apparatus and materials to prevent contamination and ensure accurate results.
- To evaporate and dry some substances eg. water from a wet apparatus.
- Initiate or speed up the chemical reaction
Most commmon sources of heat are:
- Bunsen burner
- Kerosene stove
- Spirit lamp
- Gas stove
Some of the heat sources are not commonly used in the laboratory, example:
- Electric heater
- Candle
- Charcoal burner
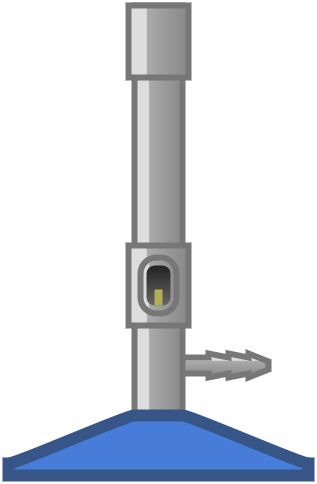
Bunsen burner
Bunsen burner is a laboratory heat source consisting of a vertical metal tube connected to a gas source.
It was invented by a Germany chemist and physicist Robert Bunsen.
It is the best of all sources of heat in the laboratory because;
- It is easy to handle
- It can produce high temperatures of about 1000°C
- Non-luminous flame is a soot-free flame.
- It is cheaper than electric heater.
- Easy to control its flame by using collar to regulate the amount of oxygen that enter the barrel.
Parts of Bunsen burner
- Barrel or chimney
- Collar or metal ring
- Jet
- Base
- Air hole
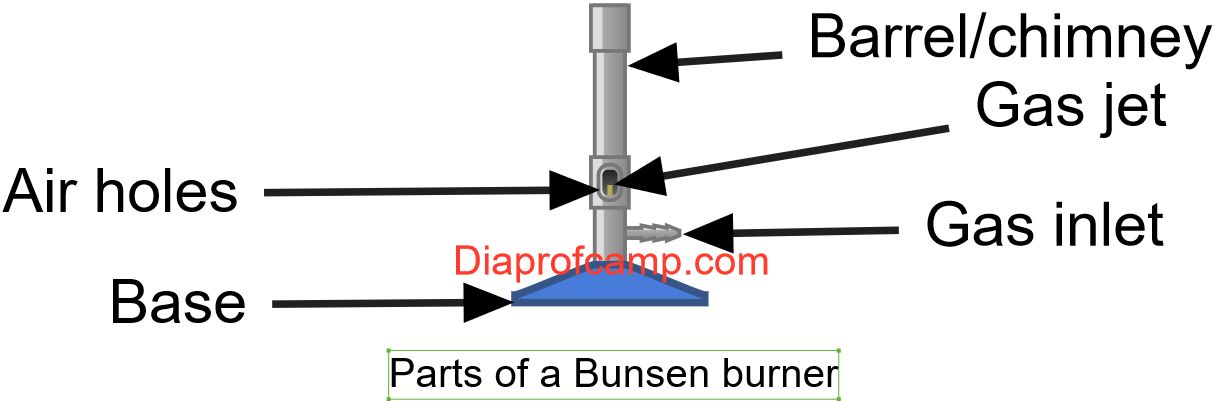
Funtions of the Different Parts of the Bunsen Burner
- The collar:- controls/regulates the amount of air entering the burner.
- The air hole - an opening which allows air to enter into the barrel of the Bunsen burner from outside.
- Barrel/chimney it is the site where gas and air mix together thoroughly ready for being burnt at the top of the burner.
- Gas jet-small hole located on the burner where the gas is released. It is narrow so that the gas enters the barrel at high pressure. Control the flow of a gas and allow it to mix with air in the barrel.
- Base - it is wide and weighty/heavy. Provides stability and prevents the Bunsen burner from tipping over or toppling.
- Gas inlet -allows the gas to enter the burner from a gas source./connects the Bunsen burner to the source of the gas.
How to Light the Bunsen burner
- Use a rubber pipe to connect the Bunsen burner to the source of gas.
- Close the air holes of the Bunsen burner.
- Light a match stick or wooden splint and bring it near the mouth of the barrel.
- Slowly while holding the match stick or wooden splint, open the gas tap.
- Open the air holes to get the non-luminous flame.
Strike back/burning back is the condition that occurs when a gas burns at the jet.
It occurs when lighting the Bunsen burner while the air holes are open.
This can be corrected by turning off the gas supply, then relighting the burner.
Generally striking back can make the gas inlet very hot, which may result to melting of the rubber tubings. This may result to the escape of the gas and cause fire explosion.
Why is not advised to turn the gas on and light the match after?
Because, if the match breaks or goes out, the gas is leaking out of the tap while you get a new match
Why we prefer closing the air holes before lighting? For safety reasons not only that yellow flame is easy to see but also air holes are closed to prevent strike back.
FLAMES
Flame is the region of burning gases that produces heat and light.
It is the visible glowing part of fire.
Bunsen burner produces two types of flame
- Luminous Flame
- Non-luminous flame
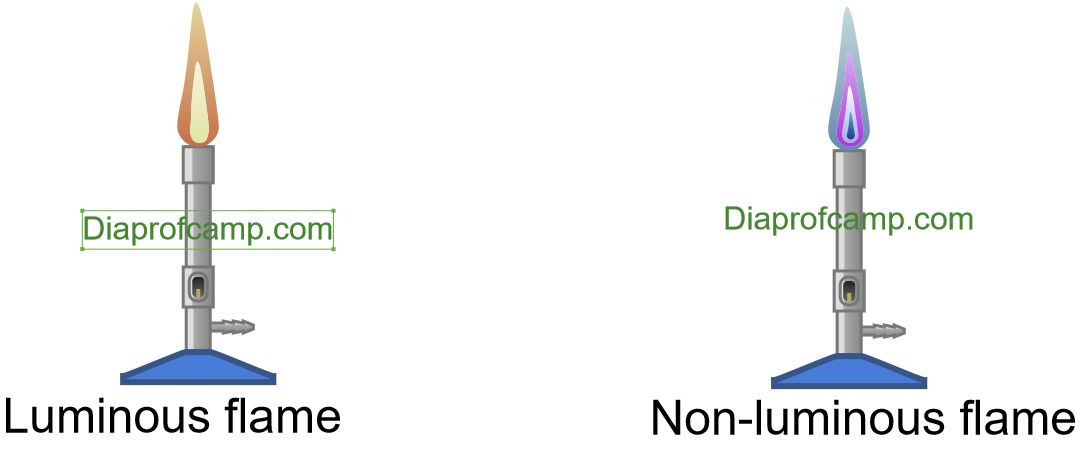
Luminous Flame
Luminous flame is bright and yellow colour. It is called luminous because it emits light.Luminous flame occurs when there is an insufficient supply of oxygen for complete combustion of fuel.
The yellow colour of the flame is due to the presence of unburned carbon particles which emit light when heated. On cooling the carbon particles form soot. Other examples of luminous flames are those produced by candle and tin lamp.
Advantages of Luminous flame
- It can be used as a source of light.
- It is called a safe flame because it is easily seen, therefore, is less likely to cause accidents.
Disadvantages of luminous flame
- It produces soot.
- It produces less heat
Parts of Luminous flame
- Thin outer zone
- Yellow middle zone
- Inner unburnt zone
- Blue outer zone.
The hottest part of luminous flame is thin outer zone because plenty of air is available for complete combustion in the outer zone.
Characteristics of a Luminous flame
It has four zones/parts.
It is not very hot.
It forms soot.
It is yellow in colour.
The flame is unsteady.
It is a quiet flame.
It is formed when air holes of Bunsen burner are closed
Non-luminous flame.
Non-luminous is the flame produced when air holes of Bunsen burner are opened. It produces more heat because there is complete combustion of carbon particles. It is called non-luminous because it gives out little light.
Parts of Non-luminous flame
It has three parts which are
- pale purple-blue zone
- blue-green middle zone
- the colourless inner zone.
Characteristics of a non-luminous flame
- It has three parts/zones.
- It is very hot.
- It does not form soot.
- It is blue or green in colour.
- It is steady.
- It is noisy.
- It is formed when air holes of the Bunsen burner are opened.
Examples of non-luminous flame is gas-cooker.
What are the differences between luminous flame and non-luminous flame.
Uses of flames
- Used to provide light at home, schools and in the areas without electricity.
A Lantern Lamp
A Transparent Glass Chimney
- Used in the laboratory experiments as source of heat.
- Used in flame test of different elements.
- Used in welding for joining together of metal pieces.
Sample of Questions
(These questions should be done by higher Forms or under teachers’ supervision)
- Mention two types of flame produced by Bunsen burner.
- Which of the two types of flame is usually used to
- heat substance to high temperatures.
- provide light when the electricity is off.
- Which part of luminous flame is the hottest of all parts?
Outermost zone of the luminous flame is the hottest flame as this part gets unlimited supply of oxygen this result to complete combustion.
- Which part of non-luminous flame is the hottest of all? Give reason to each. At the tip of the inner zone of non-luminous
- Explain the meaning of
- flame
- strike back
- luminous flame.
- Atukuzwe was told to heat a white substance T in a non-luminous flame until it turned red. She was told again to take substance R and heat it in a hot water bath until the coloured vapours evolve.
- Why she did not heat substance R on the non-luminous flame?
- What would happen if substance R was heated on the non-luminous flame?
- Draw a warning sign a substance R might has in its container.
- Madam Dorcus is a new technician at Nyagongo Secondary school. She expects to buy a source of heat, which is reliable and affordable. Besides Bunsen burner what other two sources of heat would you advise Madam Dorcus to buy? Give reasons.
- Barnaba is a new technician at Igailo Street School. He frequently uses a Bunsen burner to heat a certain chemical substance that requires high temperature.
- What type of flame do you think he uses?
- State the condition under which this type of flame you have named is produced
- Give any two advantages of using the named type of flame in the Chemistry laboratory
- Suggest two reasons for the preferences of using
- Bunsen burner in the laboratory
- Non-luminous flame during experiments




