Compounds of Metals
Metals react with other substances, especially non-metals to form different compounds.
The compounds include oxides, hydroxides, carbonates, hydrogencarbonates, nitrates, chlorides and sulphates.
A. METAL OXIDES
Metal oxide is a compound of a metal with oxygen.
Preparation metal oxides
Metal oxides can be prepared by:
- Direct method
- Indirect method
(i) Preparation of metal oxides by direct methods
In this method, a metal is directly heated in oxygen to give metal oxide.
Eg. 2Mg + O2 → 2MgO
Some metals form oxide protective layer on their surface which prevents further reactions with oxygen, example of this metal is aluminium.
(ii) Preparation of metal oxides by indirect method
This can involve preparing a metal compound and decomposing it to give out metal oxide or decomposing a compound containing oxygen.
Some hydroxides, nitrates and carbonates of moderate reactive metals such as zinc, lead, copper, iron and magnesium can be decomposed on heating.
i. Metallic carbonate
CaCO3→ CaO + CO2
ZnCO3 →ZnO + CO2
ii. Metallic nitrate
Pb(NO3)2 →PbO + NO2 +O2
iii. Metallic hydroxide
Pb(OH)2 →PbO + H2O
Classification of metal oxides
Metallic oxide can be categorized mainly into various groups:
- Basing on solubility
- Soluble metal oxides include potassium oxide, magnesium oxide, sodium oxide and barium oxide.
- Insoluble oxide include lead oxide, copper oxide etc.
- Basing on acid-base reactions
- Basic metal oxides eg. sodium, potassium and calcium oxides.
- Amphoteric oxides
These are the oxides which have basic and acidic properties. They react with bases and acids.
They include aluminium oxide,(Al2O3) lead (ii) oxide (PbO) and zinc oxide (ZnO).
Properties of metal oxides
i.Reaction with water
Soluble metal oxides react with water to form their metal hydroxides.
K2O + H2O → 2KOH
MgO + H2O →Mg(OH)2
CaO + H2O →Ca(OH)2
ii.Some metal oxides react with both acids and alkalis.
These metal oxides with such characteristics are said to be amphoteric.
- Basic properties
ZnO + 2HCl →ZnCl2 + H2O
Al2O3 + 3H2SO4 → Al2(SO4)3 + 3H2O
PbO + 3H2SO4 → PbSO4 + H2O
- Acidic properties
ZnO + 2NaOH →Na2ZnO2 + H2O
Sodium zincate
Al2O3 + 2NaOH → 2NaAlO2 + H2O
sodium aluminate
PbO + 2NaOH → Na2PbO2 + H2O
Sodium plumbate
iii.Action of heat on metal oxides
Metal oxides are stable to heat except mercury oxide which decomposes to metal and oxygen
2HgO → 2Hg + O2
Uses of metal oxides
- Soil treatment. Being basic in nature it is commonly used to treat the acidic soil. For example, calcium oxide is frequently used in this purpose.
- In the extraction of iron to remove impurities eg. Calcium oxide combines with silicon dioxide to form slag.
- Magnesium oxide and calcium oxide are used as lining of most furnaces due to their high melting points.
- Zinc oxide is used as a pigment in paints.
B. METAL HYDROXIDES
Preparation:
(i) Direct method
(ii) Indirect method
Direct method
Reactive metals such as potassium and sodium react with water to form hydroxide and hydrogen gas
2K + 2H2O → 2KOH + H2
Metals below hydrogen in the reactivity series cannot be used to prepare hydroxide by direct method.
Indirect method
Under this method, alkali reacts with salts such as chlorides, sulphates and nitrates to form hydroxide.
ZnCl2 + 2NaOH → 2NaCl + Zn(OH)2.
CuSO4 + 2NaOH → Cu(OH)2 + Na2SO4.
Classification of metal hydroxides
- Basing on solubility
Alkali metal hydroxides are very soluble in water while alkaline earth metal hydroxides are sparingly soluble in water. Other metal hydroxides are insoluble in water.
- Basing on acid-base reactions
Hydroxides of aluminium, zinc and lead are amphoteric.
Pb(OH)2 + 2NaOH →Na2PbO3 + 3H2O
Other hydroxides are basic hydroxide.
Chemical properties
- Amphoteric metal hydroxides, that is Pb(OH)2, Zn(OH)2, Al(OH)3 react with both acids and bases.
- Metal hydroxides react with acids to form salt and water only.
Ca(OH)2 + HCl → CaCl2 + H2O
- All hydroxide except potassium hydroxide and sodium hydroxide decompose under heat to produce oxide and steam.
4. Mg(OH)2 (s)→ MgO(s)+ H2O(g)
Uses of metal hydroxides
- Used in soil treatment eg. calcium hydroxide
- aluminium and magnesium hydroxides are used as antacids to neutralize stomach acid.
- sodium hydroxide is used in the extraction of aluminium from bauxite ore.
- calcium hydroxide is used to remove temporary hardness of water.
- sodium hydroxide is used in the identification of some cation in salt sample during qualitative analysis.
C. METAL CARBONATES AND HYDROGENCARBONATES
Metal carbonates and hydrogencarbonates are derived from carbonic acid (H2CO3).
(A) METAL CARBONATES
Metal carbonates are formed when both hydrogen atoms in carbonic acid are replaced by a metal. It is therefore a salt.
Preparation of metal carbonates
- Soluble metal carbonates can be prepared by reaction of metal hydroxide solution with carbon dioxide.
2NaOH + CO2 → Na2CO3 + H2O
2KOH + CO2 →K2CO3 + H2O
- Insoluble carbonates can be prepared by a precipitation reaction
ZnSO4 + Na2CO3 → ZnCO3 + Na2SO4
Classifications of metal carbonates
Metal carbonates can be classified based on solubility, whereby potassium and sodium carbonates are soluble in water. Other metal carbonates are insoluble in water.
Aluminium and iron (III) carbonates do not exist.
Chemical properties of metal carbonates
Action of heat on metal carbonates.
Carbonates of potassium and sodium are stable to heat, that is, they do not decompose on heating.
The other metal carbonates decompose to give the metal oxide and carbon dioxide.
PbCO3 → PbO + CO2
CuCO3 → CuO + CO2
Action of dilute acids on metal carbonate.
All metal carbonates react with dilute mineral acids to give carbon dioxide gas.
ZnCO3 + HCl → ZnCl2 + H2O + CO2
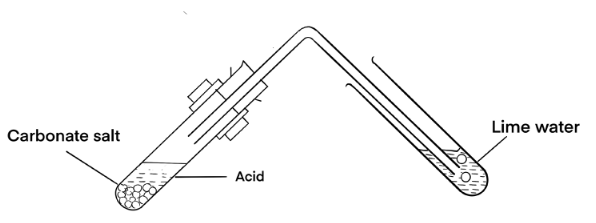
TESTS FOR CARBONATES
- Dilute acid test.
When dilute acid is added to a solid or solution of suspected carbonate, effervescences of carbon dioxide gas evolve.
CO32-(aq) + 2H+(aq) → CO2(g) + H2O(l)
When carbon dioxide is passed through lime water, limewater turns milky.
- Magnesium sulphate test.
This test is performed in the case of soluble carbonate only.
Soluble carbonates react with the magnesium sulphate solution to form white precipitate of magnesium carbonate.
Na2CO3(aq) + MgSO4(aq) → MgCO3(s)(white ppt) + Na2SO4(aq).
- Barium chloride test.
This solution can be also used to test soluble carbonates.
BaCl2(aq) + Na2CO3(aq) → BaCO3(s)(white ppt) + 2NaCl(aq).
(B) METAL HYDROGENCARBONATES
Metal hydrogencarbonates are formed when only one of two hydrogen atoms in the carbonic acid (H2CO3) is replaced with a metal ion.
Hydrogencarbonates of metals lower than magnesium in the reactivity series do not exist.
Preparation of metal hydrogencarbonates by
Passing excess carbon dioxide on concentrated hydroxide solution.
NaOH(aq) + CO2 (g)→ Na2CO3(aq) + H2O(l)
Then
Na2CO3 + CO2 + H2O → 2NaHCO3
The same reaction can occur to the limewater (Ca(OH)2)
Chemical properties of hydrogencarbonates
- Action of heat on hydrogencarbonates
All hydrogencarbonate decompose on heating to give carbon dioxide and carbonate.
NaHCO3 → Na2CO3 + H2O + CO2
- Action of acids on hydrogencarbonates
2KHCO3 + H2SO4 → K2SO4 + 2CO2 + 2H2O
TESTS OF HYDROGENCARBONATES
Magnesium sulphate test:
Magnesium sulphate solution reacts with hydrogencarbonate on heating to produce white precipitates.
What happens is:
2NaHCO3(aq) → Na2CO3(aq) + H2O(l) + CO2(g) (on warming/heating)
Then
Na2CO3(aq) + MgSO4(aq) → Na2SO4 (aq)+ MgCO3(s)(white ppt)
Always remember this!
Magnesium suphate solution has no effect on hydrogencarbonate in the absence of heat.
MgSO4(aq) + 2NaHCO3 (aq)→ Na2SO4(aq) + Mg(HCO3)2(aq).
Uses of carbonates and hydrogencarbonates
- Softening of hard water eg. Na2CO3
- Manufacturing of glass.
- Sodium hydrogencarbonate is used to cure indigestion.
- Calcium carbonate is used to form calcium oxide which is used to neutralize the excess acidity in the soil.
D.METAL NITRATES
Metal nitrates are metal salts of nitric acid formed by displacement of the hydrogen ion of the acid by a metal.
Preparation of nitrates
Metal nitrates are usually prepared by methods that involve crystallization. This can be done by reacting a metal, carbonate, an oxide or alkali with dilute nitric acid.
Examples
NaOH + HNO3 → NaNO3 + H2O
CaCO3 + 2HNO3 → Ca(NO3)2 + H2O + CO2
PbO + 2HNO3 → Pb(NO3)2 + H2O
Mg + HNO3 → Mg(NO3)2 + H2
Chemical properties of metal nitrates
Action of heat on nitrates
Thermal stability of metal nitrates can be linked to the position of the metal in the reactivity series.
K
Na |
Nitrates of these metals decompose to nitrite and oxygen.
MNO3 → MNO2 + O2
Example
2NaNO3 → 2NaNO2 + O2
|
Ca
Mg
Al
Zn
Fe
Pb
Cu |
The nitrates of these metals decompose to oxide, nitrogen dioxide and oxygen gas.
MNO3 → MO + NO2 + O2
Example
2Zn(NO3)2 → 2ZnO + 4NO2 + O2
|
Hg
Ag |
Nitrates of these metals decompose to metal, nitrogen dioxide and oxygen.
MNO3 → M + NO2 + O2.
Example
2AgNO3 → 2Ag + 2NO2 + O2
|
The letter M stands on behalf of a metal
The nitrates of lowly reactive metals (Hg, Ag) can even be easily decomposed by sunlight. This is why they are kept in brown/dark glass bottles.
Tests of nitrates
1. Copper turnings test
To a small solid sample of metal nitrate copper turnings are added followed by concentrated sulphuric acid, the mixture is then heated/warmed.
. Brown fumes evolve, indicating the presence of nitrate ions.
Example
2NaNO3 + 4H2SO4 + 3Cu → 3CuSO4 + Na2SO4 + 4H2O + NO2
The brown fumes evolve is nitrogen dioxide gas, NO2
2. Brown ring test
To aqueous solution of metal nitrate, ferrous sulphate (FeSO4) is added and then concentrated sulphuric acid is added along the sides of the test tube dropwise. This forms a layer on the top of the liquid already present in the test tube.
. Brown ring is formed at the junction of the liquids. This indicates that the nitrate ions are present.
The following reactions take place:
i. NaNO3 + H2SO4 → NaHSO4 + HNO3
ii. 6FeSO4 + 3H2SO4 + 2HNO3 → 3Fe2(SO4)3 + 4H2O + 2NO
iii. FeSO4 + NO → FeSO4.NO (dark brown)
Uses of metal nitrates
1.In agriculture it is used as nitrogeneous fertilizer.
2.They are used in food preservation.
E.METAL CHLORIDES
Metal chlorides are metal salts containing chloride ions. They are formed when the hydrogen of hydrochloric acid (HCl) is replaced by a metal.
Preparation of metal chlorides
By direct method
Chlorides can be prepared by direct action of chlorine on metals.
Aluminium chloride and iron (III) chloride can be made by this method.
This is what happens:
- Dry chlorine gas is passed over heated iron or aluminium. Since the reaction is exothermic, the reaction can continue without application of heat. Once the reaction begins, the heat source can be turned off/ or removed.
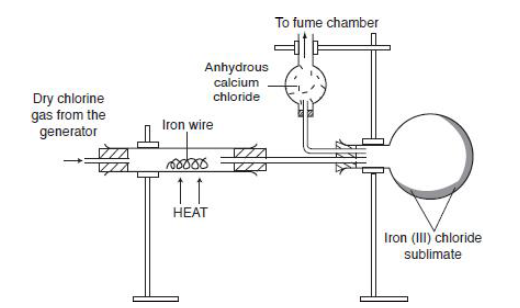
- Iron (III) chloride forms as a vapour and solidifies in cold parts of the jar.
- Calcium chloride absorbs moisture, so it keeps the apparatus dry.
2Fe + 3Cl2 → 2FeCl3
2Al + 3Cl2 → 2AlCl3
Anhydrous iron(III) chloride is dark brown. If left in air it absorbs moisture and turns yellow. It has changed to hydrated iron(III) chloride, with the formula FeCl3.6H2O.
By indirect method
Soluble chlorides can be prepared by reacting dilute hydrochloric acid with
- Oxides
- Hydroxides
- Carbonates
- Metals
| Chlorides of: | Can be prepared by |
|---|
K
Na
Ca | action of the oxide, hydroxide or carbonate on dilute hydrochloric acid. |
Mg
Al
Zn
Fe | Action of the metal, oxide and carbonate on dilute hydrochloric acid |
NOTE
The reaction between dilute hydrochloric acid and lead (II) carbonate forms insoluble salt (lead (II) chloride) which forms coating around the carbonate and stops further reactions.
Chemical properties of chlorides
Action of heat on metal chlorides
Generally, chlorides do not decompose on heating.
However, hydrated chlorides undergo hydrolysis
MgCl2.6H2O → Mg(OH)2 + 2HCl + 4H2O
TESTS OF CHLORIDES
1. To identify a chloride ion in a solution add lead nitrate or silver nitrate.
A white precipitate indicates the presence of the chloride ion.
Lead and silver chlorides are the only common insoluble chlorides.
Pb(NO3)2(aq) + NaCl(aq) → PbCl2(s) + NaNO3(aq)
AgNO3(aq) + NaCl (aq)→ AgCl (s) + NaNO3(aq)
2.For solid chloride samples
(a)Add concentrated sulphuric acid to the suspected solid chloride. Heat the mixture.
Colourless gas with pungent smell evolves and the gas gives dense white fumes with ammonia solution due to the formation of ammonium chloride.
NaCl(s) + H2SO4(l) → NaHSO4(l) + HCl(g)
HCl + NH3(aq) → NH4Cl (white dense fumes)
This indicates the presence of Cl- ions in the salt sample.
(b) Mix a chloride with an oxidizing agent eg. manganese(IV) oxide, add concentrated sulphuric acid and warm.
→A greenish yellow gas evolves, that turns damp/wet blue litmus paper red and then bleaches it. The gas is chlorine.
2NaCl + MnO2 + 2H2SO4 → Na2SO4 + MnSO4 + 2H2O + Cl2
Uses of chlorides
- Sodium chloride is used to add taste to food.
- Zinc chloride and ammonium chloride are used in dry batteries
- Sodium chloride is used in the food preservation
F. METAL SULPHATES
Sulphates are salts that are derived from sulphuric acid.
Preparation of sulphates
(a) Soluble sulphates are prepared by reacting a metal oxide, a metal carbonate, a metal hydroxide or a metal with dilute sulphuric acid and obtaining the crystals by crystallization.
CuO + H2SO4 → CuSO4 + H2O
NaOH + H2SO4 → Na2SO4+ H2O
ZnCO3 + H2SO4 → ZnSO4 + H2O + CO2
Zn + H2SO4 → ZnSO4 + H2
(b) Insoluble sulphates can be prepared by adding dilute sulphuric acid to solutions containing lead or barium ions.
BaCl2(aq) + H2SO4 (aq)→ BaSO4(s) + 2HCl(aq)
Pb(NO3)2(aq) + H2SO4(aq) → PbSO4(s) + HNO3(aq)
Then the insoluble salts can be filtered and dried.
NOTE
The reaction between sulphuric acid and calcium /lead/barium carbonates stops after a short time due to the formation of an insoluble layer of calcium/lead/barium sulphates which tend to form coatings around the carbonates and stop further reactions.
Chemical properties of metal sulphates
1. Action of heat
In general sulphates are more stable to heat. The stability however depends on the position of a given metal in the reactivity series.
K
Na
Ca
Mg | The sulphates of these metals are stable to heat |
Moderate reactive metals, their sulphates decompose when strongly heated to oxides and sulphur dioxide or trioxides.
CuSO4.5H2O → CuSO4 + 5H2O (on gentle heating.)
CuSO4 → CuO + SO3 (strong heating).
ZnSO4 → ZnO + SO3 (strong heating).
FeSO4.7H2O → FeSO4 + 7H2O
2FeSO4 → Fe2O3 + SO3 +SO2
Fe2(SO4)3.9H2O→ Fe2(SO4)3 + 9H2O
Fe2(SO4)3 → Fe2O3 + 3SO3
2. Metal sulphates react with acids in a double decomposition reaction.
Test for sulphates
The suspected aqueous solution is acidified with hydrochloric acid followed by addition of barium chloride solution.
The white precipitate appears. This confirms the presence of sulphate ions.
BaCl2(aq) + Na2SO4(aq) → BaSO4(s)+ NaCl(aq)
Ba2+ (aq) + SO42- (aq) → BaSO4(s)
Uses of sulphate
- Aluminium sulphate is used in urban water treatment and purification.
- Copper sulphate is used as fungicides.
- Plaster of paris is used for plastering fractured bones and dislocated bones so as to set them in proper place.

