Ammonia
Ammonia is a compound of hydrogen and nitrogen (NH3).
Laboratory preparation of ammonia
It is prepared in the laboratory by heating ammonium chloride with calcium hydroxide.
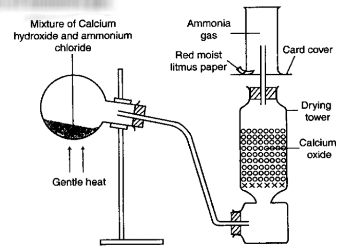
Laboratory preparation of ammonia
NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + 2NH3
→The round bottomed flask is tilted (kept in an inclined position) to prevent any condensed water from running back into the hot flask, which would make the flask crack.
→ Calcium oxide (CaO) is used as a drying agent for the gas because other drying agents react with ammonia.
2NH3 + H2SO4 → (NH4)2SO4
6NH3 + P2O5 + 3H2O → 2(NH4)3PO4
4NH3 + CaCl2 → CaCl2.4NH3
→ Ammonia gas is collected by downward displacement of air because it is less dense than air.
→Ammonia cannot be collected by downward displacement of water, because it is extremely soluble in water.
Another method of preparing ammonia is by heating magnesium nitride (Mg3N2) with water.
Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH3
PROPERTIES OF AMMONIA
(a) Physical properties of ammonia
- Ammonia is a colourless gas with a pungent choking smell.
- Ammonia turns wet red litmus paper blue showing that the gas in alkaline in nature.
- Ammonia is less dense than air.
- Ammonia is highly soluble in water. This can be illustrated by using the Fountain Experiment.
(b) Chemical properties of ammonia
- Reaction with water.
Ammonia gas dissolves in water to form ammonia solution.
NH3(g) + H2O(l) → NH4+(aq) + OH-(aq).
- Reducing property of ammonia
Ammonia reduces copper (II) oxide (CuO) and lead (II) oxide (PbO) to the respective metals and oxidizes itself to nitrogen gas.
2NH3 (g)+ 3CuO(s) → N2(g) + 3Cu(s) + 3H2O(l)
2NH3(g) + PbO (s)→ N2(g) + 3Pb(s) + 3H2O(l)
- Reaction with concentrated hydrochloric acid
NH3 + HCl → NH4Cl (dense white fumes)
- Reaction with oxygen
(a)On burning:
4NH3 + 3O2 → 2N2 + 6H2O
(b) Under platinum catalyst
4NH3 + 5O2 → 4NO + 6H2O
(Further oxidation of oxide above, reddish brown gas evolves
NO(g) + O2 (g) → NO2(g))
- Reaction with chlorine
2NH3 + 3Cl2 → N2 + 6HCl
In excess of NH3
8NH3 + 3Cl2 → 6NH4Cl + N2
Test for ammonia gas
It form a dense white fumes with concentrated hydrochloric acid or hydrogen chloride gas.
Uses of ammonia
- It is used in the manufacture of nitric acid.
- It is used in the manufacture of nitrogeneous fertilizers eg. ammonium sulphate and urea
- It is used in the softening of hard water.
- As refrigerant
